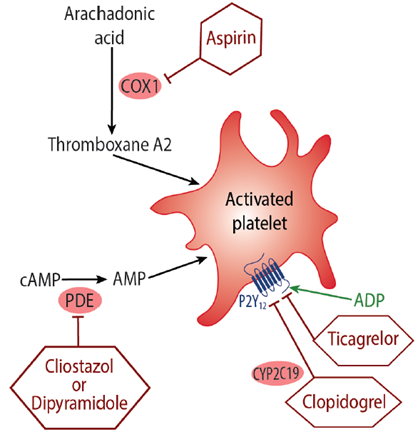
Anti-platelet drugs attenuate platelet aggregation and inhibit thrombus formation, and are used for the prevention of atherothrombotic and thromboembolic events.18 Two of the most commonly used therapies are aspirin and adenosine-diphosphate (ADP) receptor antagonists, such as clopidogrel, ticagrelor and prasugrel (Figure 2).
Aspirin exerts its anti-platelet effect by inhibiting cyclooxygenase-dependent platelet aggregation and reducing the synthesis of the platelet activator thromboxane A2.19,20 It is one of the most widely used drugs in the treatment and prevention of cardiovascular disease, and the efficacy of aspirin in secondary prevention of cardiovascular events is well established.21,22 However, long-term use has been associated with an increased risk of major haemorrhage21 and gastrointestinal bleeding.22,23
Clopidogrel, ticagrelor and prasugrel are antagonists of the P2Y12 receptor, which inhibits ADP-induced platelet aggregation and also platelet activation, preventing the formation of blood clots.24 Clopidogrel is indicated for the prevention of atherosclerotic events in at-risk patients, such as those with prior myocardial infarction or ischaemic stroke.25 Whilst effective at preventing cardiovascular morbidity, clopidogrel has been associated with an increase in major and minor bleeding.26