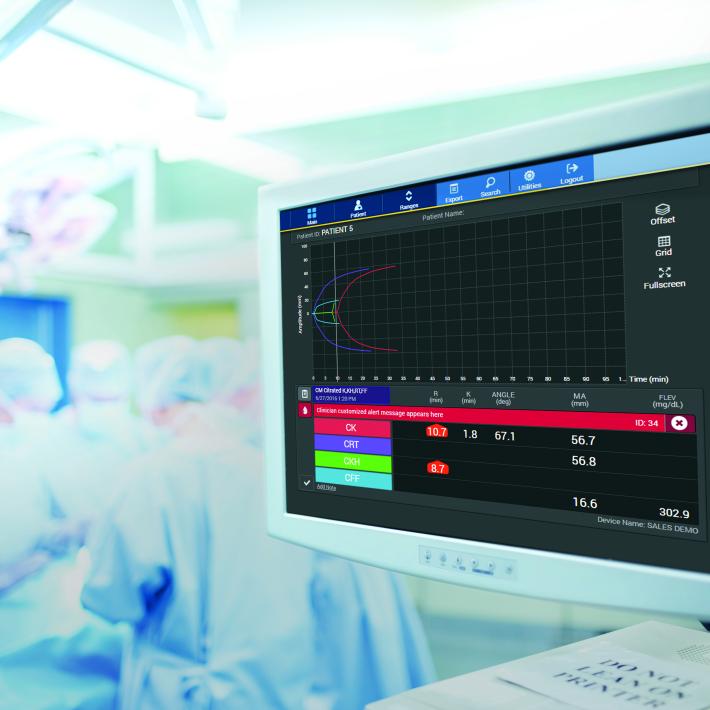
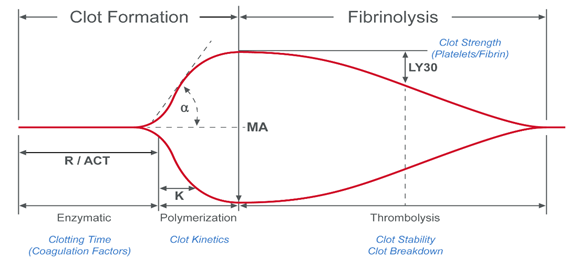
During the process of coagulation, blood experiences radical changes that cause it to lose viscosity and become more elastic.1 The resulting blood clots are able to resist deformation under shear forces, an ability called shear modulus (or modulus of rigidity). In VHA systems, the shear modulus (also known as clot stiffness) is measured either directly or indirectly to give an indication of the coagulation status of a blood sample.
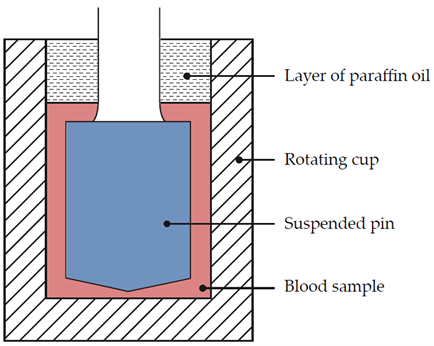
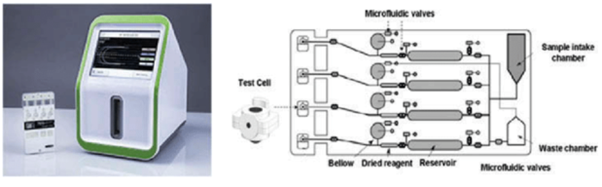
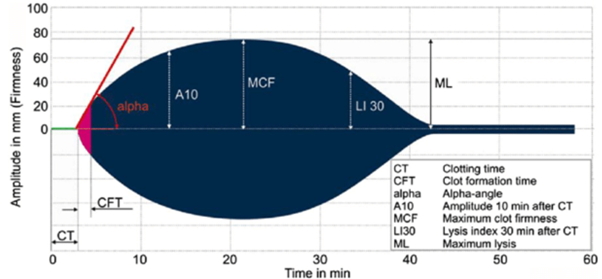
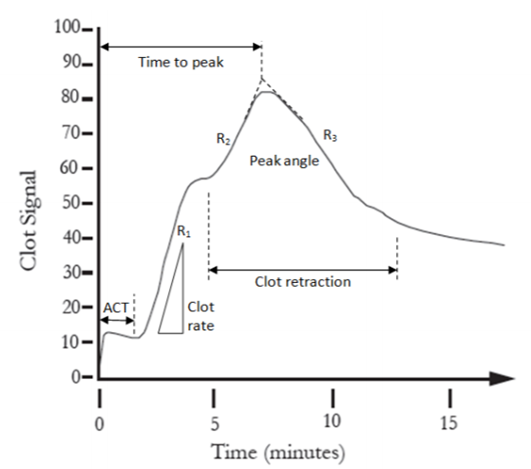
Dr Hellmut Hartert was the first to develop a mechanism to quantify the dynamics of blood clot formation, called viscoelastic haemostatic monitoring, in 1948 (Figure 1).2 The primary measurement of viscoelastic haemostatic monitoring is the observation of blood transforming from a viscous to elastic state, with VHAs assessing whole blood clotting dynamics by measuring the speed in which a blood sample can form a clot.2 By adding different reagents, investigators can determine the relative contribution of platelets, coagulation factors or fibrinogen (in cases of inadequate clotting).2 Importantly, VHAs measure the entire dynamic change of a clot and provide an overview of the haemostatic state of a blood sample, rather than just showing the presence or absence of individual coagulation factors.3